
The Hanarostent® Benign is a unique class of technology that allows minimizes the development of granulation tissue and is outer silicone coating to block the direct contact between mucosa and nitinol wire. It is the flange type with thicker silicone membrane coated onto the outside of metal mesh. This membrane thickness is 0.2-0. 24 mm. The distance between end of metal mesh and end of silicone membrane is 5 mm. |
 |
Indications for use (Intended use)The HANAROSTENT® Esophagus BS Benign is indicated for palliative treatment of esophageal malignant and/or benign stricture and trachea-esophageal fistula. |
 |
Using Hanarostent® Benign, no tissue overgrowth is observed makes it possible to leave the stent in place for several months and exchange it easily, contrary to other stents.1
1. Frederic Prat, Hepato-gastroenterology Department, Hospital Cochin, Comparison of a standard fully covered stent with a super- thick silicone-covered stent for the treatment of refractory esophageal benign strictures: A prospective multicenter study, DOI: 10.1177/ 2050640613476501
Animal study between a conventional stent and a new stent for the benign stricture2
Six dogs with the weight of 8~10kg have been prepared and grouped into three. Three different types of metallic stents have been inserted into each group. Tissue reaction has been observed every 2 weeks until the week of 8th by endoscope. After 8th week, the gross tissue was reviewed by pathologist.
| Conventional Stent | Hanarostent® Benign |
|---|---|
| Endoscopic View
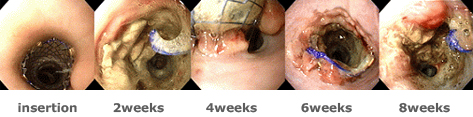
Tissue response has started to develop at the 2nd week from the insertion of a conventional stent. At the 4th week, granulation tissue was found and the stent became completely enbeded into the esophageal mucosa at the 6th week. At the 8th week, massive granulation tissue resulted in partial esophageal stricture. | Endoscopic View
Tissue response has started to develop at the 2nd week from the insertion of a conventional stent. At the 4th week, granulation tissue was found and the stent became completely enbeded into the esophageal mucosa at the 6th week. At the 8th week, massive granulation tissue resulted in partial esophageal stricture. |
 |
 |
| Pathologic findings of conventional self expandable metal stent
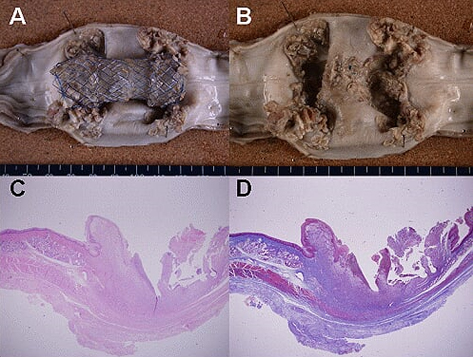
Conventional stent group. An artificial stent is impacted and adhered to the esophageal wall(A). There is large ulceration and marked exuberant granulation tissue at the both proximal and distal side of stent(B). Microscopic finding demonstrate large mucosal defect, marked granulation tissue and collagenous fibrosis, loss of submucosal glands and proper muscle layer(C, H-E, x12.5; D, Masson's trichrome, x12.5). |
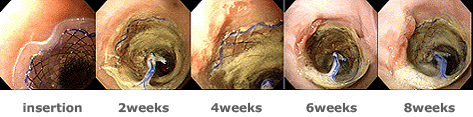
Pathologic findings of newly designed self expandable metal stent - flange type
Newly designed SEMS - flange type. A stent is noted within the esophagus(A). There is focal ulceration with small amount of granulation tissue and focal mucosal regeneration after the removal of the stent (B). Microscopic finding demonstrate focal small ulcer, marked submucosal thickening by granulation tissue and fibrosis, focal loss of muscle layer, epithelial hyperplasia, and residual small amount of submucosal glands(C, H-E, x12.5; D, Masson's trichrome, x12.5). |
 |
 |
| Stent(mm) | Delivery Device | ||||
|---|---|---|---|---|---|
| Diameter | Usable Length* | Total Length | Length(mm) | Diameter(mm/fr) | |
| EBN18060-Z070 | 24-18-24 | 20 | 60 | 700 | 8/24 |
| EBN18120-Z070 | 80 | 120 | 700 | 8/24 | |
| EBN20060-Z070 | 26-20-26 | 20 | 60 | 700 | 8/24 |
| EBN20120-Z070 | 80 | 120 | 700 | 8/24 | |
| EBN22060-Z070 | 28-22-28 | 20 | 60 | 700 | 8/24 |
| EBN22120-Z070 | 80 | 120 | 700 | 8/24 | |
※Sizing and availability varies by country
All medical devices have associated risks. Please refer to the package insert and other labeling for a complete list of indications, contraindications, precautions and warnings. For further information on the Products, please contact your local M.I.Tech(Hanarostent) Representative.