Introduction
6Fr-introducer enhances maneuverability in narrowing per-hilar bile duct and side by side simultaneous-bilateral endoscopic deployment with “Benefit™”, closed cell type
Indications for use (Intended use)
The Non-Covered Biliary Stent is used for application in palliative treatment of bile duct stricture caused by malignant tumors.
Product Overview
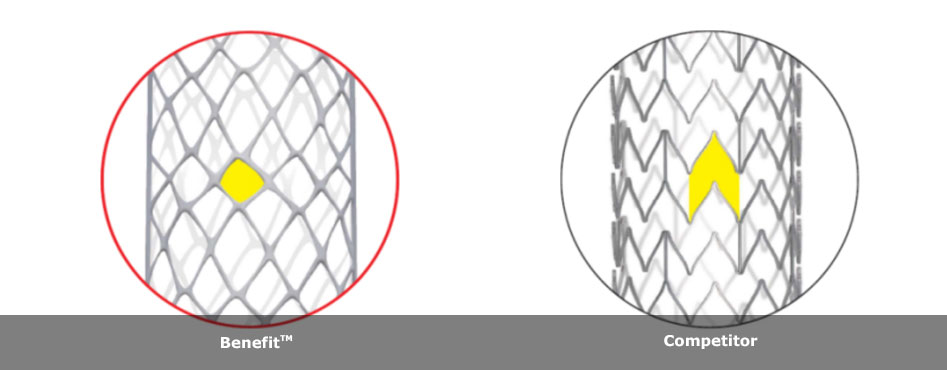 |
|
| 6Fr-introducer | 6Fr-introducer provides ease of use and maneuverability in tight bile duct. |
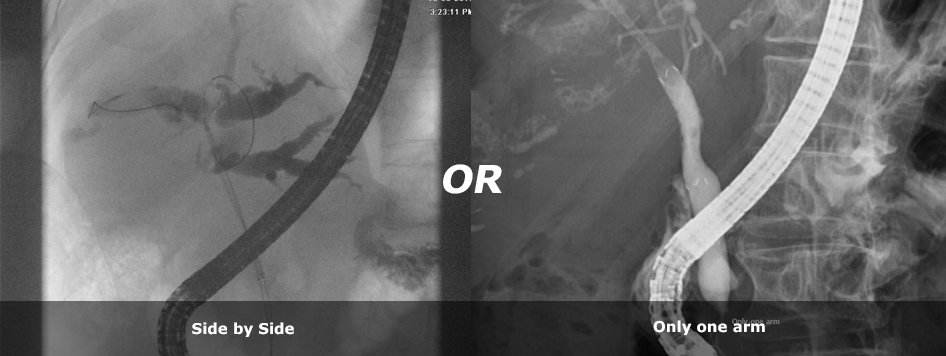 |
|
| "Seok Jeong, MD,Ph.D., Division of Gastroenterology, Department of Internal Medicine, Inha University School of Medicine” | |
Resources
ORDERING INFO.
Endoscopic Application
*Increment of 10mm| Stent(mm) | Delivery Device | ||||
|---|---|---|---|---|---|
| Diameter | Usable Length* | Total Length | Length(mm) | Diameter(mm/fr) | |
| BNHS-06-040-180 | 6 | 26 | 40 | 1800 | 1.97/5.9 |
| BNHS-06-120-180 | 106 | 120 | 1800 | 1.97/5.9 | |
| BNHS-08-040-180 | 8 | 26 | 40 | 1800 | 1.97/5.9 |
| BNHS-08-120-180 | 106 | 120 | 1800 | 1.97/5.9 | |
Percutaneous Application
| Stent(mm) | Delivery Device | ||||
|---|---|---|---|---|---|
| Diameter | Usable Length* | Total Length | Length(mm) | Diameter(mm/fr) | |
| BNHS-06-040-060 | 6 | 26 | 40 | 600 | 1.97/5.9 |
| BNHS-06-120-060 | 106 | 120 | 600 | 1.97/5.9 | |
| BNHS-08-040-060 | 8 | 26 | 40 | 600 | 1.97/5.9 |
| BNHS-08-120-060 | 106 | 120 | 600 | 1.97/5.9 | |
RESOURCES
※Sizing and availability varies by country
All medical devices have associated risks. Please refer to the package insert and other labeling for a complete list of indications, contraindications, precautions and warnings. For further information on the Products, please contact your local M.I.Tech(Hanarostent) Representative.