
Alternative option for the treatment of obstructive jaundice when endoscopic biliary drainage is not achievable and end-bare cover metallic stents for Endoscopic ultrasound-guided hepaticogastrostomy (EUS-HGS)
The Non-Covered Biliary Stent is used for application in palliative treatment of bile duct stricture caused by malignant tumors.
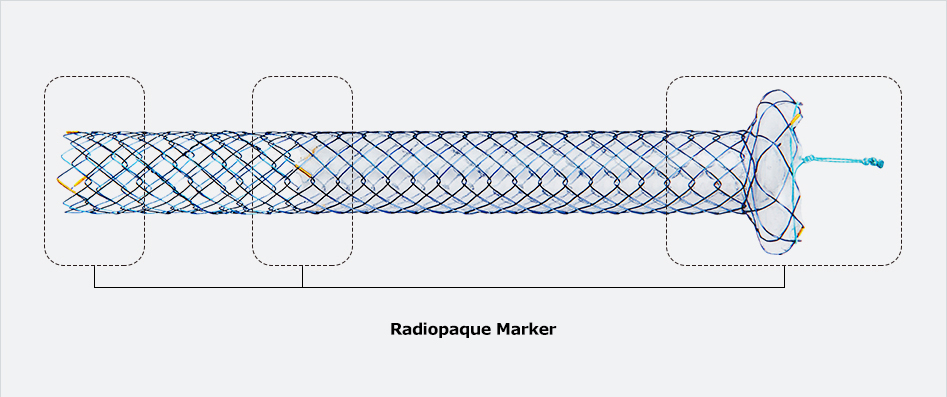
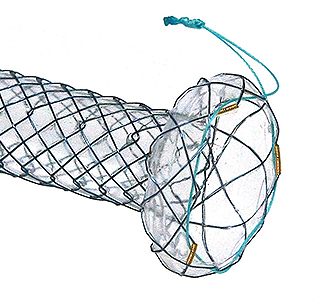 Flange head prevents migration into the hepatlc duct
Flange head prevents migration into the hepatlc duct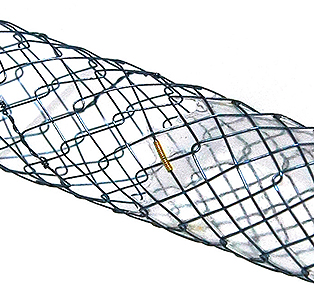 Covered prevents the bile leakage from hepatlc duct
Covered prevents the bile leakage from hepatlc duct Uncovered prevents migration In hepatlc duct and avolds blocking the side branches
Uncovered prevents migration In hepatlc duct and avolds blocking the side branches| Stent(mm) | Delivery Device | ||||
|---|---|---|---|---|---|
| Diameter | Usable Length* | Total Length | Length(mm) | Diameter(mm/fr) | |
| BPD10060-E180 | 10-20 | 54 | 60 | 1800 | 2.83/8.5 |
| BPD10080-E180 | 74 | 80 | 1800 | 2.83/8.5 | |
| BPD10100-E180 | 94 | 100 | 1800 | 2.83/8.5 | |
※Sizing and availability varies by country
All medical devices have associated risks. Please refer to the package insert and other labeling for a complete list of indications, contraindications, precautions and warnings. For further information on the Products, please contact your local M.I.Tech(Hanarostent) Representative.